Current Clinical Trials

We are running a phase 2b clinical trial for Idiopathic Pulmonary Fibrosis (IPF) patients with chronic cough called CORAL (COugh Reduction in IPF with nALbuphine ER ). The CORAL trial is a double-blind, randomized, placebo-controlled, parallel-arm study evaluating three doses of Haduvio (27mg, 54mg and 108mg twice daily) in IPF patients with chronic cough.
Approximately 160 IPF patients with chronic cough are expected to be randomized 1:1:1:1 to one of three Haduvio doses or placebo for a period of 6-weeks, which includes a 2-week titration period followed by 4-weeks of fixed dose.
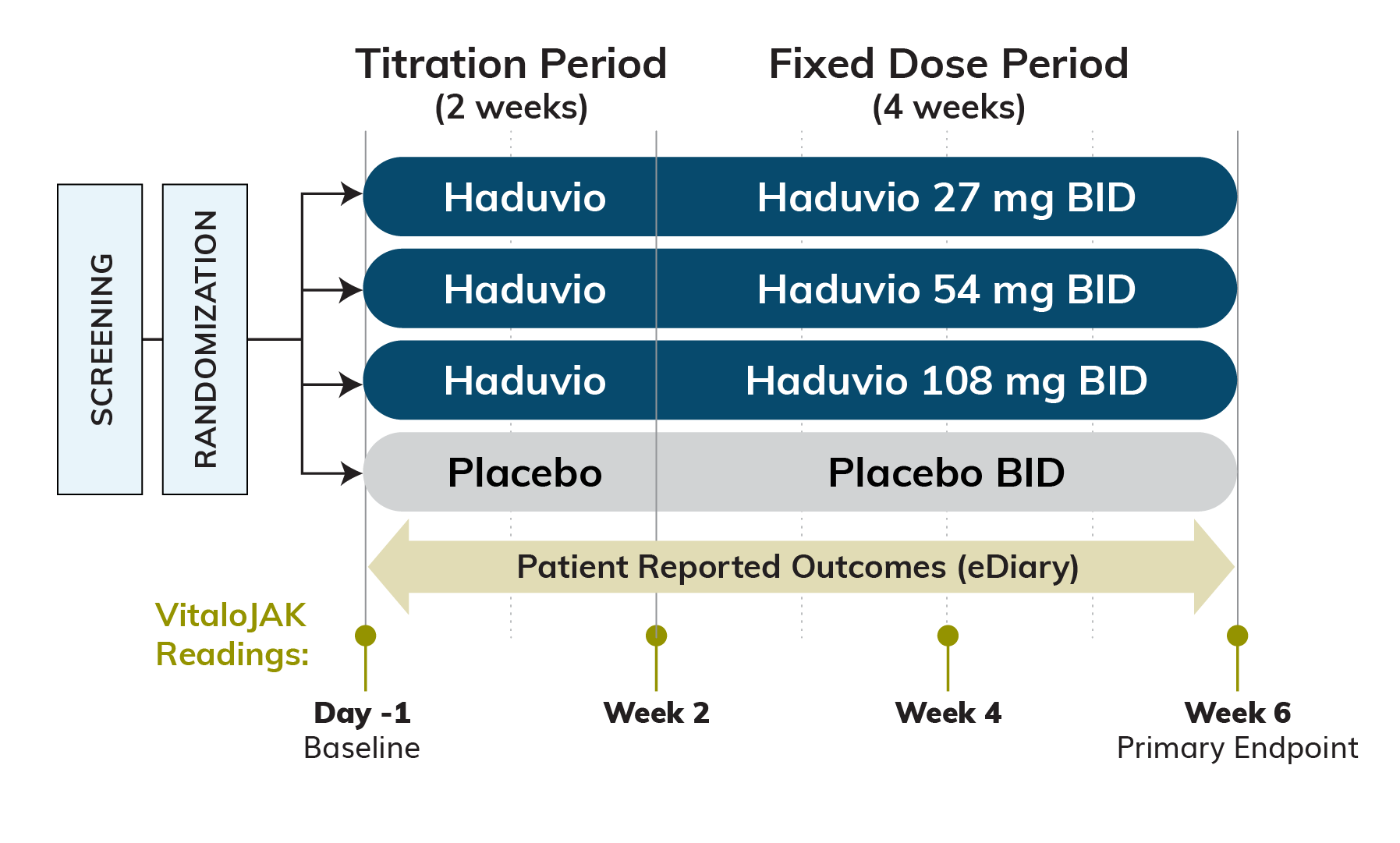
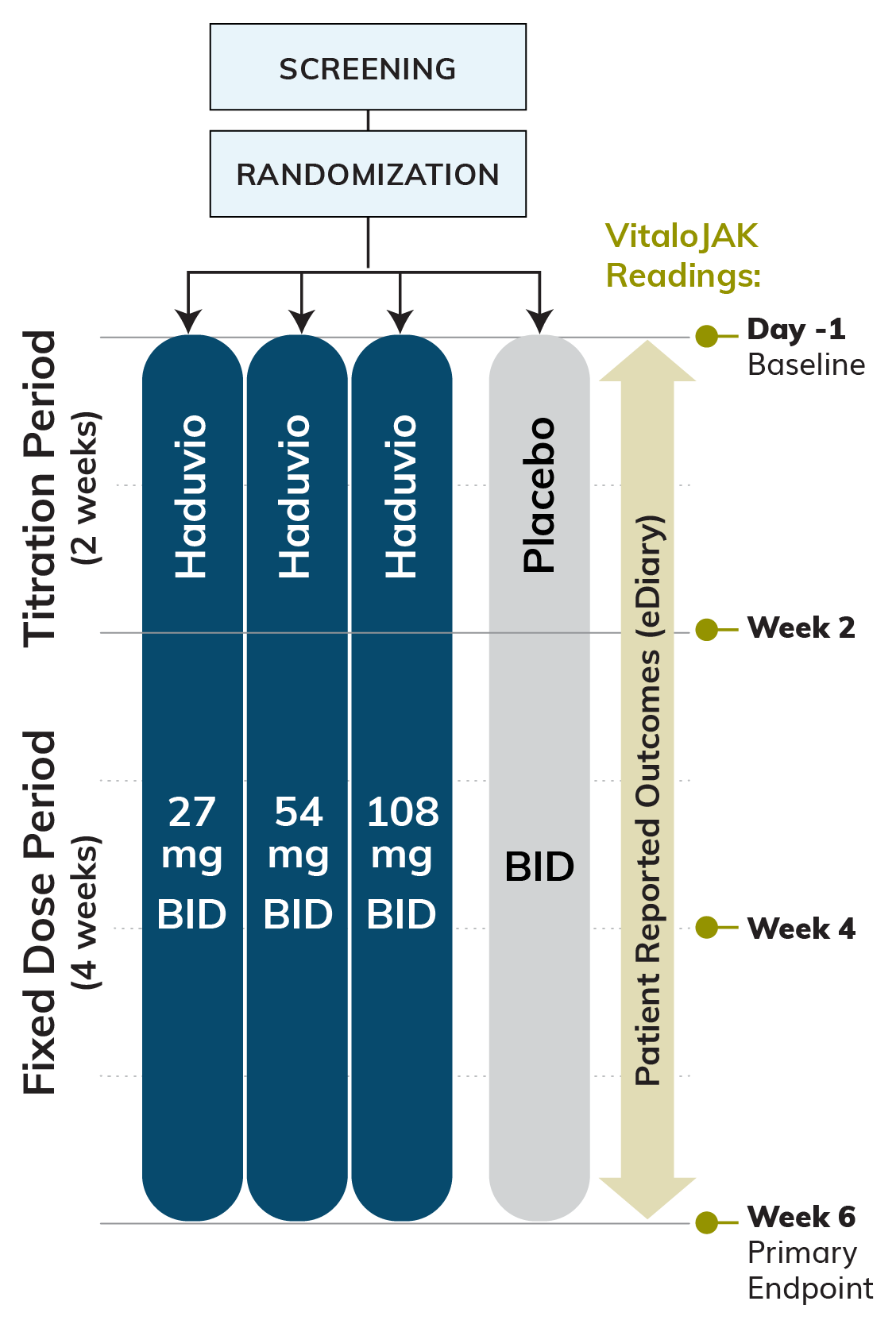
Primary Efficacy Endpoint:
Relative change in 24-hour cough frequency at the end of Week 6 versus baseline for Haduvio compared to placebo, as measured via an objective cough monitor.
Expected Timing:
- Sample size re-estimation 2H 2024
- Topline data 1H 2025
“…there’s no ability to stop it or control it, it’s like it takes over, it just takes over your body and until that cough is out… there’s no way to stop it or control it”
– RCC/UCC Patient
Kum E et al. 2022 doi:10.1183/23120541.00667-2021

Approximately 60 RCC subjects are expected to be randomized with a 1:1 stratification strategy between those with 10-19 coughs/hour (moderate 24-hour cough frequency) and those with ≥20 coughs/hour (high 24-hour cough frequency). Each treatment period will last 21 days, separated by a 21-day washout period, and subjects on Haduvio will have the dose titrated from 27 mg once a day (QD) up to 108 mg twice a day (BID) across the 21-day dosing period.
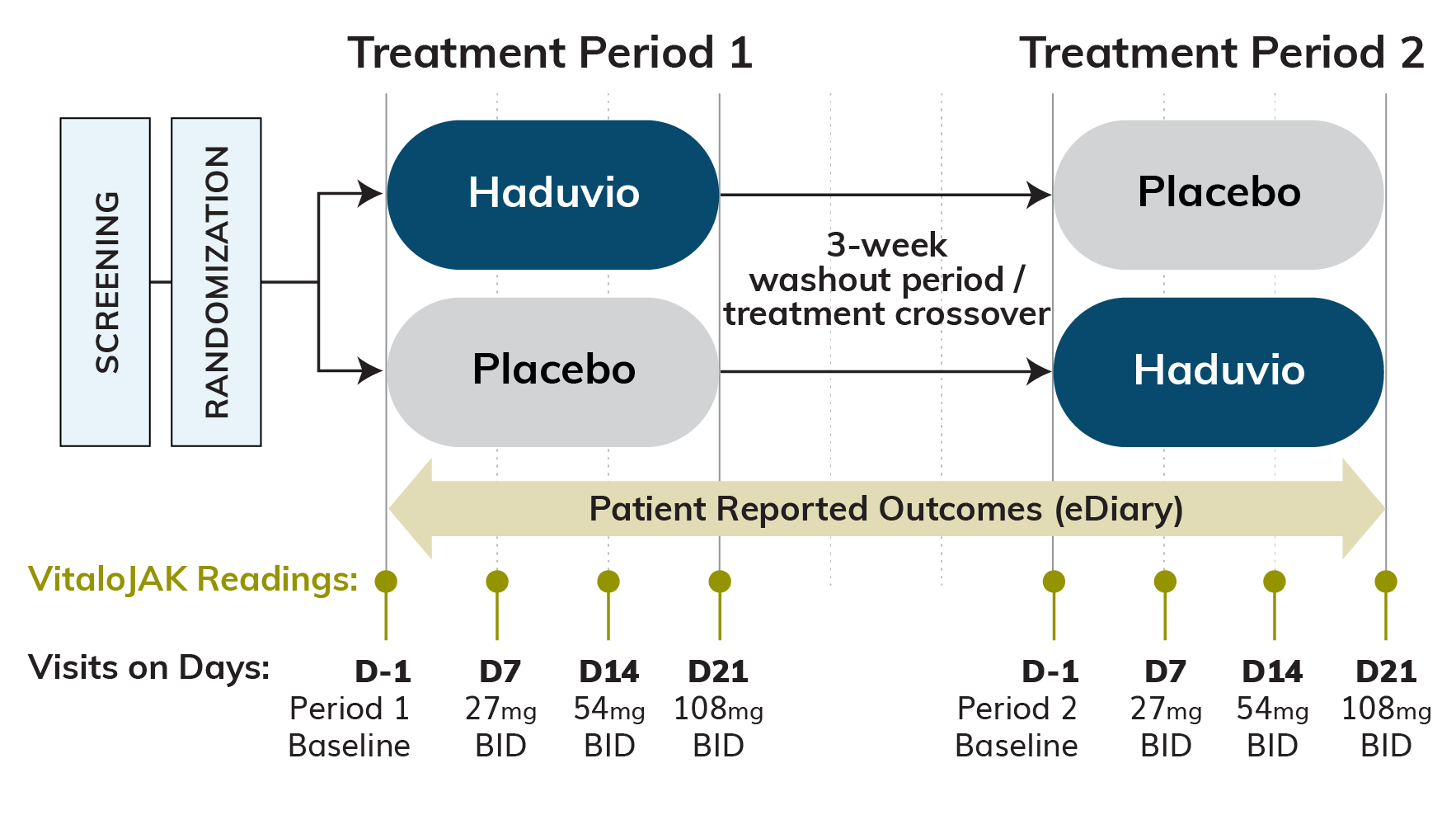
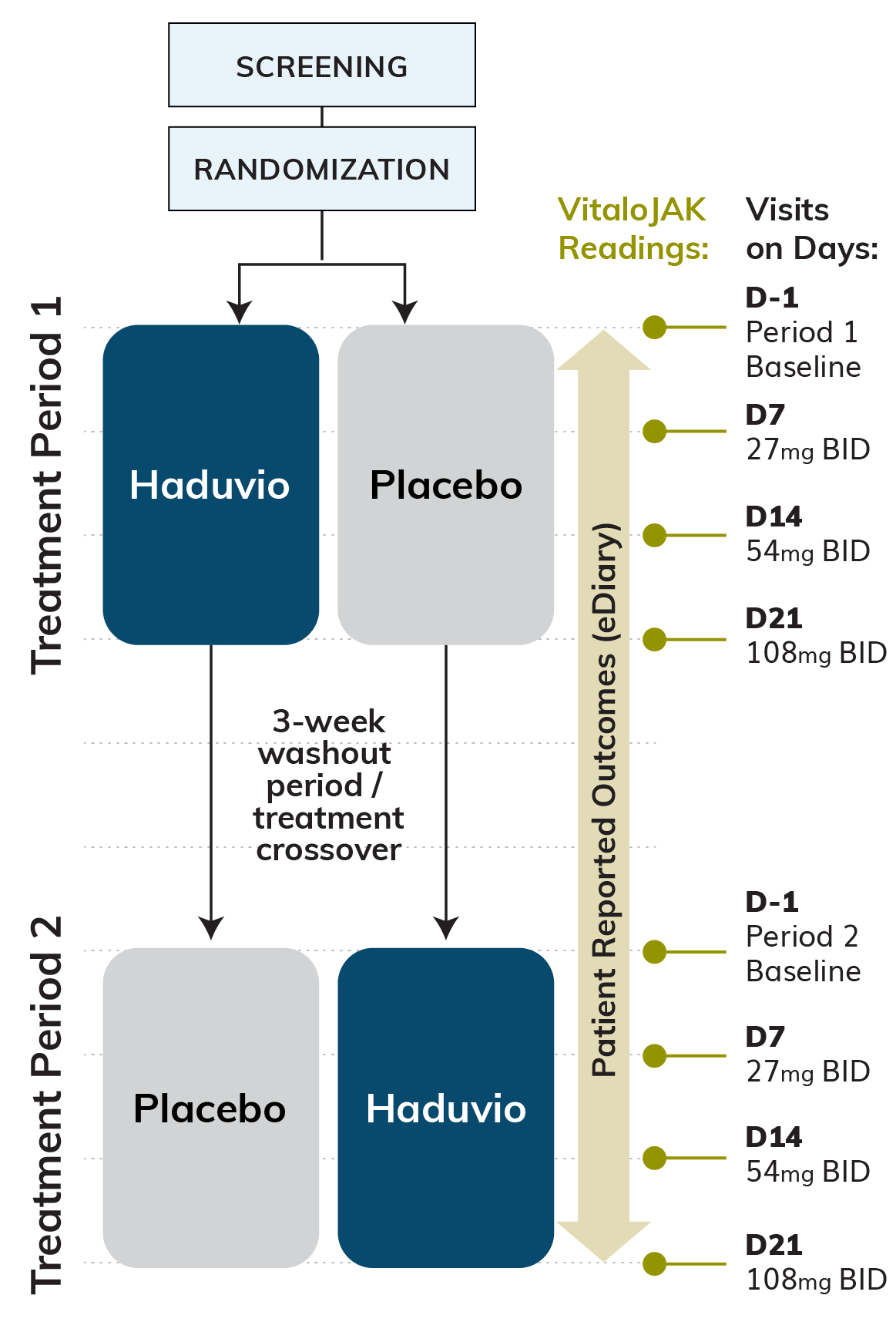
Primary Efficacy Endpoint:
Relative change in 24-hour cough frequency at Day 21 from baseline for Haduvio compared to placebo, as measured via an objective cough monitor.
Expected Timing:
- Topline data 2H 2024*
*Expected topline data from CORAL dependent on SSRE results.
Human Abuse Potential Trial
We initiated a human abuse potential, or HAP, trial in the fourth quarter of 2022 to compare the abuse potential of oral nalbuphine to intravenous, or IV, butorphanol. The study is a randomized, double-blind, active and placebo-controlled 5-way crossover design and is conducted in two parts. We completed the first part of the HAP trial and have gained FDA agreement on the proposed butorphanol dose to be used in the second part of the trial. The second part of the trial will initiate dosing in January 2024 with the primary objective of evaluating the likability of nalbuphine as compared to both placebo and butorphanol.
Topline Data Expected 2H 2024
Topline Data Expected 2H 2024
Future Clinical Trials
We plan to initiate a phase 1b respiratory physiology trial in IPF patients.
