Previous Clinical Trial Results
Haduvio™ – The First and Only Investigational Oral Therapy to Achieve Primary Endpoint in Treating Chronic Cough in IPF
We are currently developing Haduvio™ (oral nalbuphine ER) to help patients suffering from serious chronic cough conditions. We completed a Phase 2a Trial for adults with chronic cough in idiopathic pulmonary fibrosis and are planning further development in IPF, as well as future development in refractory chronic cough.
%
of IPF Patients Experience Chronic Cough

Phase 2a Cough and NALbuphine (CANAL) Trial
Geometric Mean Percent Change from Study Baseline in Coughs per Hour
Full Analysis Set (N=38)
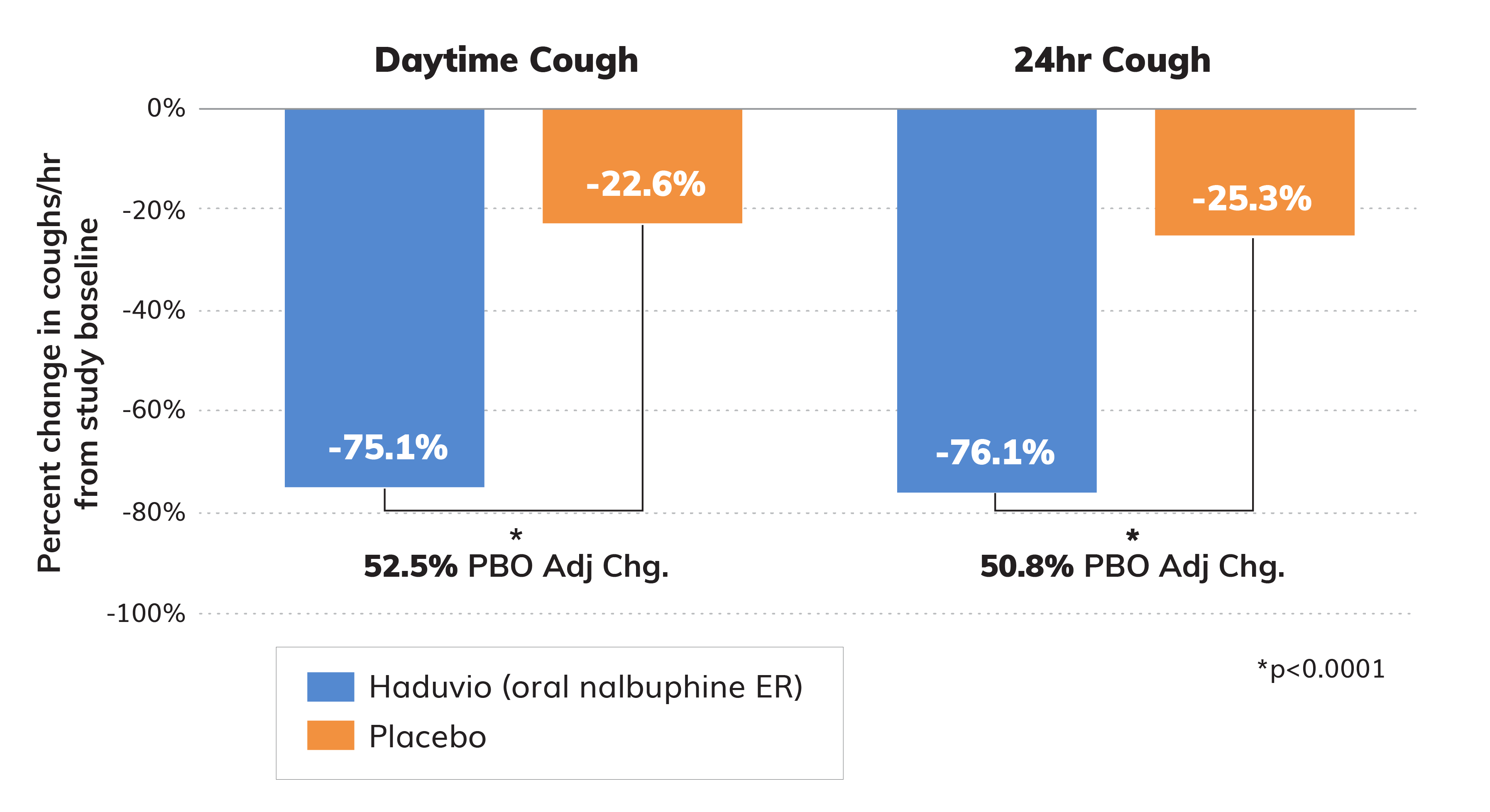
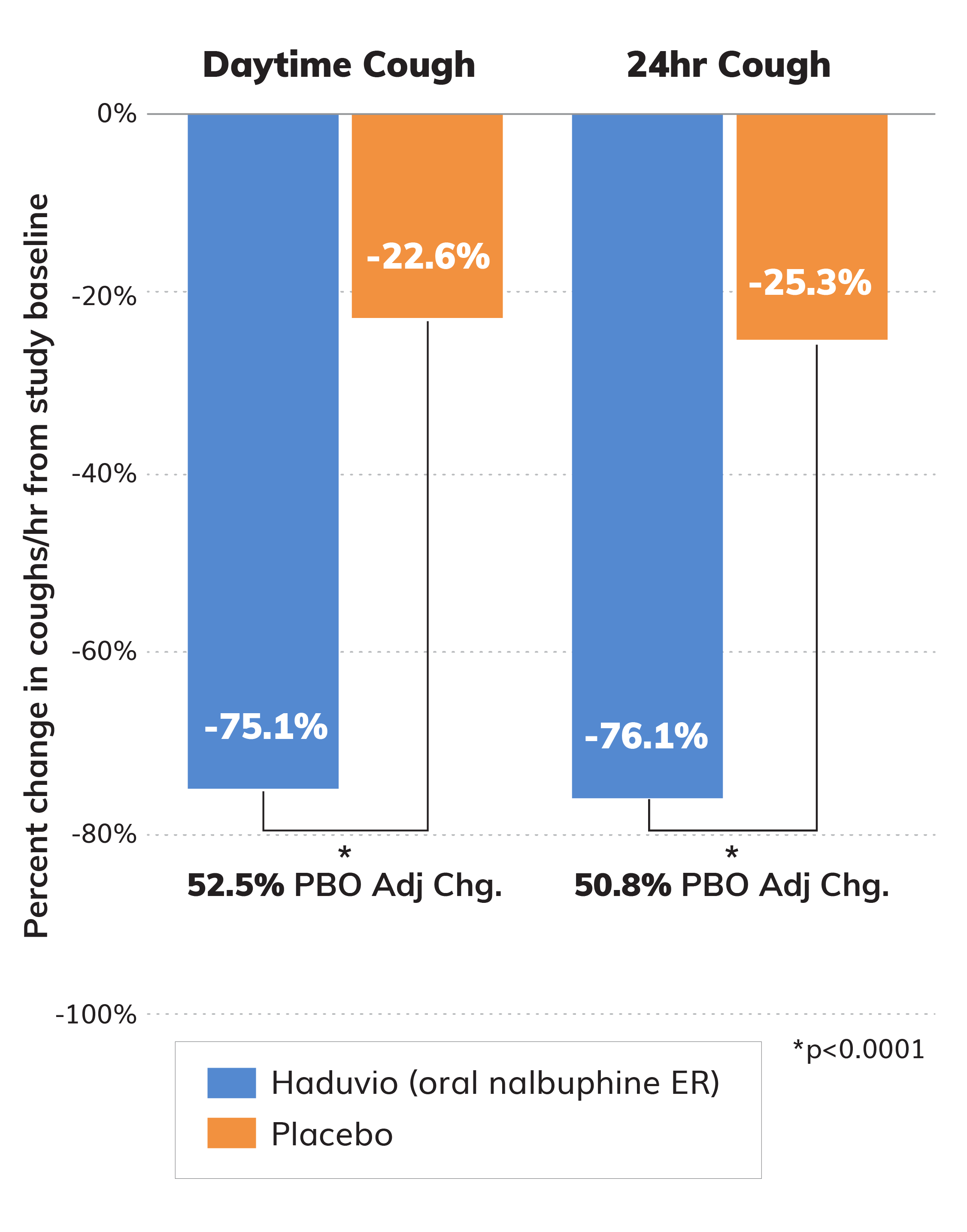
Topline data from the full set of subjects (N=38) in the Phase 2 CANAL trial for adults with chronic cough in IPF showed a 75.1% reduction in daytime cough frequency for those on Haduvio.
We also measured cough reduction over a 24-hour period and the data also demonstrated a significant reduction in cough similar to the daytime measurement.
Responders from Study Baseline
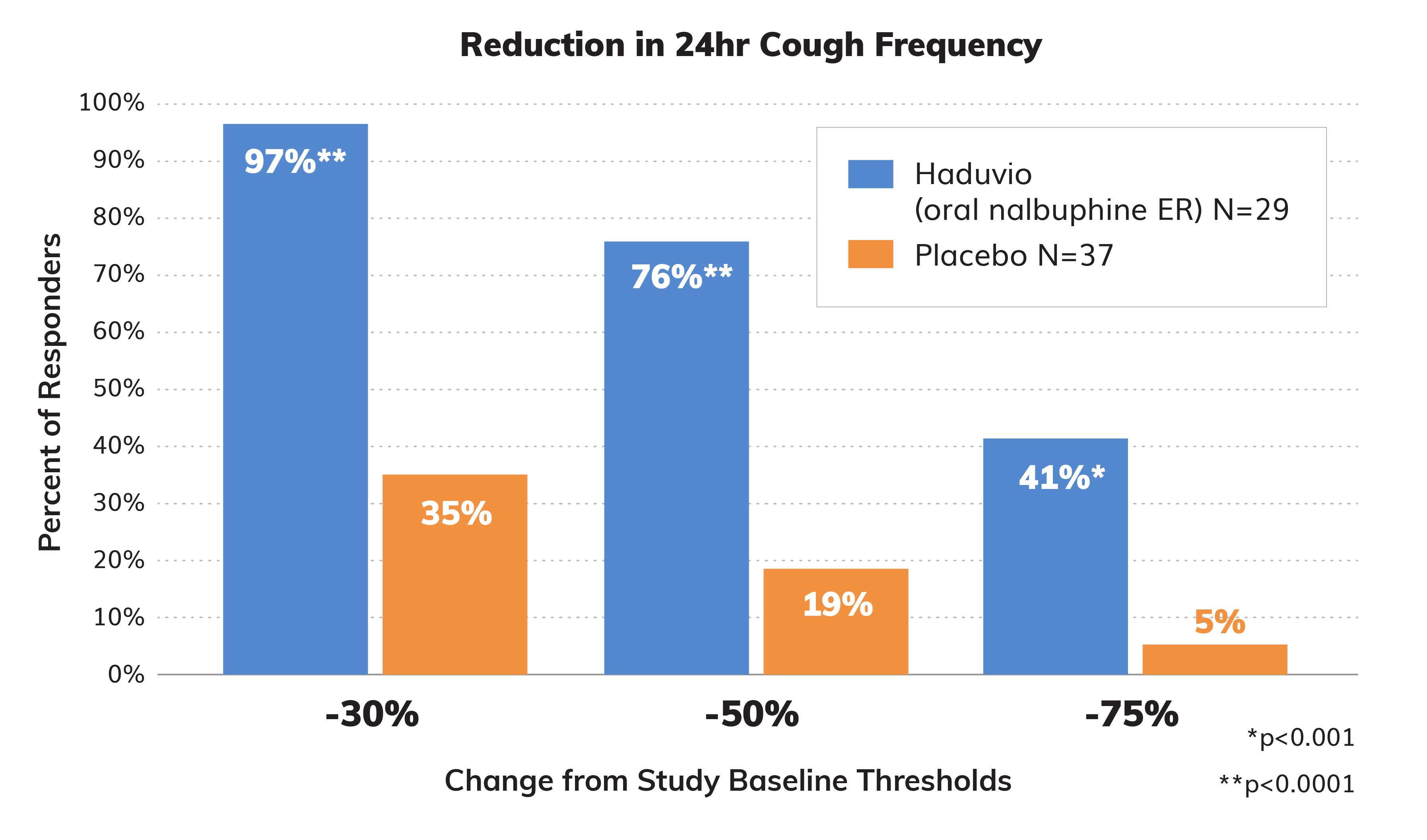
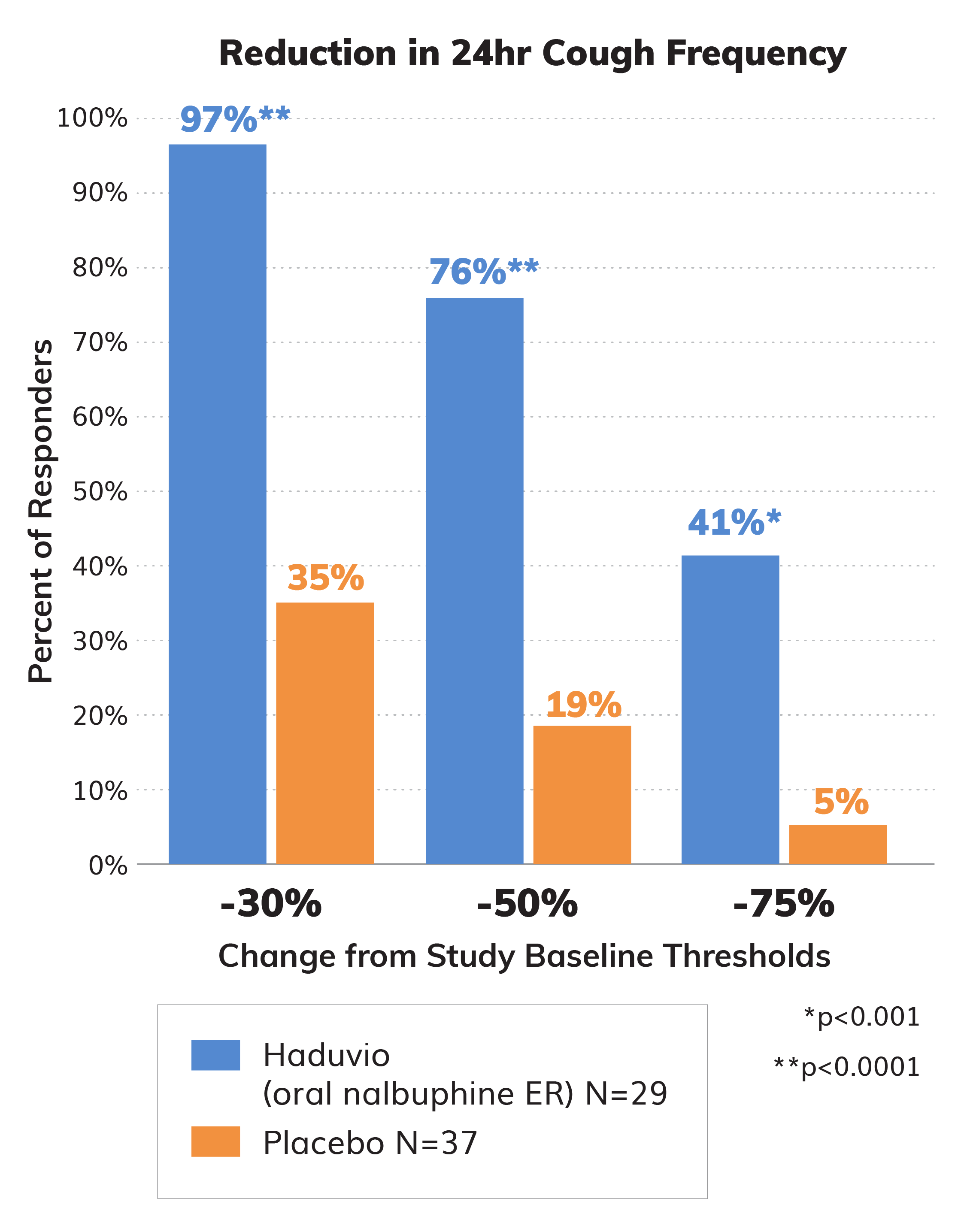
The strength of the CANAL data was also analyzed by the percentage of subjects that experienced a 30%, 50%, and 75% reduction in their 24-hour cough frequency. A 20-30% reduction in cough frequency is considered to be clinically meaningful and 97% of Haduvio subjects saw at least a 30% reduction in 24-hour cough frequency.
The safety results of the trial were generally consistent with the known safety profile of Haduvio from previous trials. Adverse events most commonly observed during the trial were nausea, fatigue, constipation, dizziness, somnolence, vomiting, headache, anxiety and depression.
Current Clinical Trials
Based on the strength and consistency of these results, we initiated a Phase 2a trial in refractory chronic cough and a Phase 2b trial in IPF patients with chronic cough.
